
Though misidentified as a lead compound with selenium and iron components, the material was in fact lead chromate with a formula of PbCrO 4, now known as the mineral crocoite. On 26 July 1761, Johann Gottlob Lehmann found an orange-red mineral in the Ural Mountains which he named Siberian red lead. With this, you complete the procedure of growing alum.6, 5, 4, 3, 2, 1 (strongly acidic oxide) After you get the alum of the desired size, remove it from the jar. If, by chance, the crystal, has touched the sides or the bottom of the jar, then pour the liquid from the jar in which you are growing the crystals into another jar, follow the same procedure mentioned above and allow the crystal to grow. To fix it properly, you will require a few attempts.Ĩ. Tie it in such a way that the alum is in the liquid (alum solution), but it does not touch the sides or the bottom of the jar. The other end should be tied to a flat object like a scale, knife, pen, pencil, etc. To achieve this, tie a nylon fishing line around the seed crystal. While growing the big crystals, the main aspect that should be considered is that the seed crystal should not touch the sides or the bottom of the jar. These alum crystals are known as seed crystals and they are further used for growing big crystalsħ. You will observe small crystals at the bottom of the jar after the water has been poured out.Ħ. After the stipulated amount of time mentioned above, pour the water to another jar.ĥ. Keep this mixture undisturbed for at least 12 hours.Ĥ. To serve this purpose, you can cover the jar with a paper towel or a coffee filter. Keep this mixture away from dust and other contaminants. Add the alum slowly and keep stirring continuously till the alum dissolves in water.ģ. In a clean jar, pour in ½ cup of hot water.Ģ. This alum can be represented chemically as Na 2SO 4.Al 2(SO 4) 3.24H 2O. Soda alum is also referred to as sodium alum. It is also used in the preparation of soda. Soda alum is nothing but mendozite the mineral which is found naturally in the environment. This type of alum is crystalline in nature and violet in color. This double sulfate of potassium and chromium is represented as KCr(SO 4) 2.12H 2O. Unlike other types of alum, chrome alum is used in the process of tanning. Minerals like kalinite and alunite are natural sources of potash alum. Potash alum is also widely used as a water purifying agent. Potash which is also referred to as aluminum potassium sulfate and is used as an astringent and antiseptic. This type of alum is crystalline in nature and white in color. This alum is a double sulfate of aluminum. Ammonium alumĬhemically, ammonium alum can be represented as NH 4Al(SO 4) 2.12H 2O. There are four different types of alum, namely ammonium alum, potash alum, chrome alum and soda alum. Heat causes them to liquefy and further heating leaves salt froth as residue which then swells and forms a powder which is amorphous in nature.These compounds are hydrates i.e they contain moisture or water molecules that are trapped in the solid structure. Their general formula is AM(SO4) 2.12H 2O, where A is a monovalent cation such as potassium or sodium and M is a trivalent cation like aluminium or chromium. Alum is also a class of compounds which are formed when alkali and trivalent metals react with each other.It is known to possess haemostatic and antibacterial properties.Ayurveda describes the antiseptic, anti-inflammatory, antipyretic, and antibiotic properties of alum.These particles either settle down at the bottom or float on it which can easily be removed by filtration or sedimentation.

Alum acts as a flocculating agent which coagulates all the contaminants and pollutants present in the water.
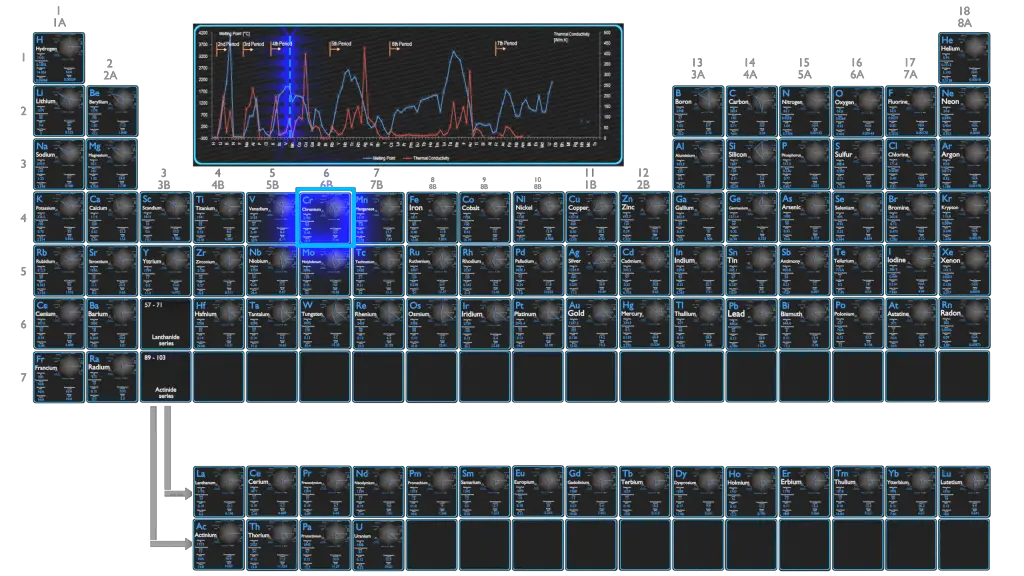
The most commonly used alum is the potasssium alum with the chemical formula KAl(SO 4) 2.12H 2O.Bauxite is also a source from which alum can be prepared. Alum is prepared from alunite which is a sulfate mineral.


 0 kommentar(er)
0 kommentar(er)
